- SPP2191 of the DFG
2022 – 2024
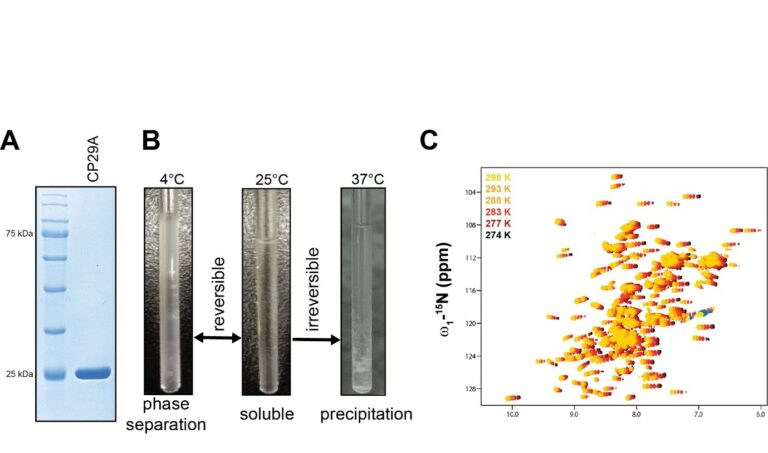
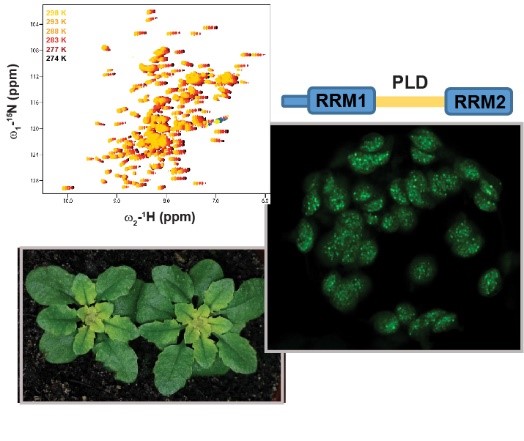
- Background: Plants as sessile organisms cannot run when challenged by environmental stress, including temperature stress. Instead, they have developed a plethora of sophisticated physiological and molecular reactions to maintain homeostasis. […]
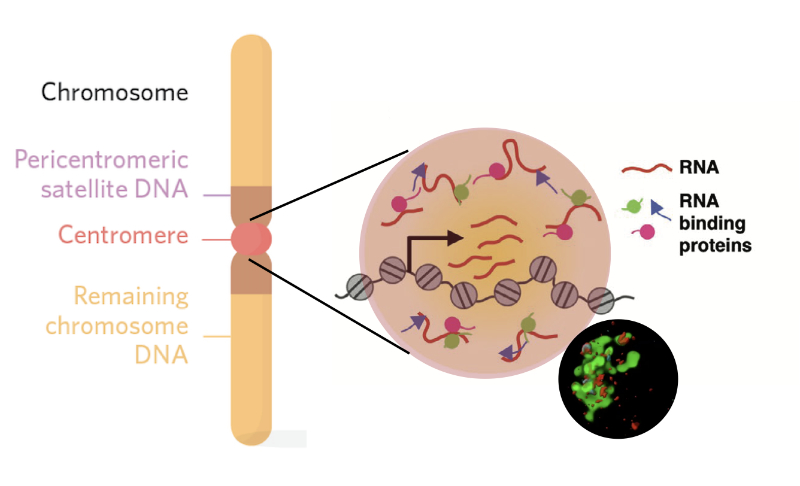
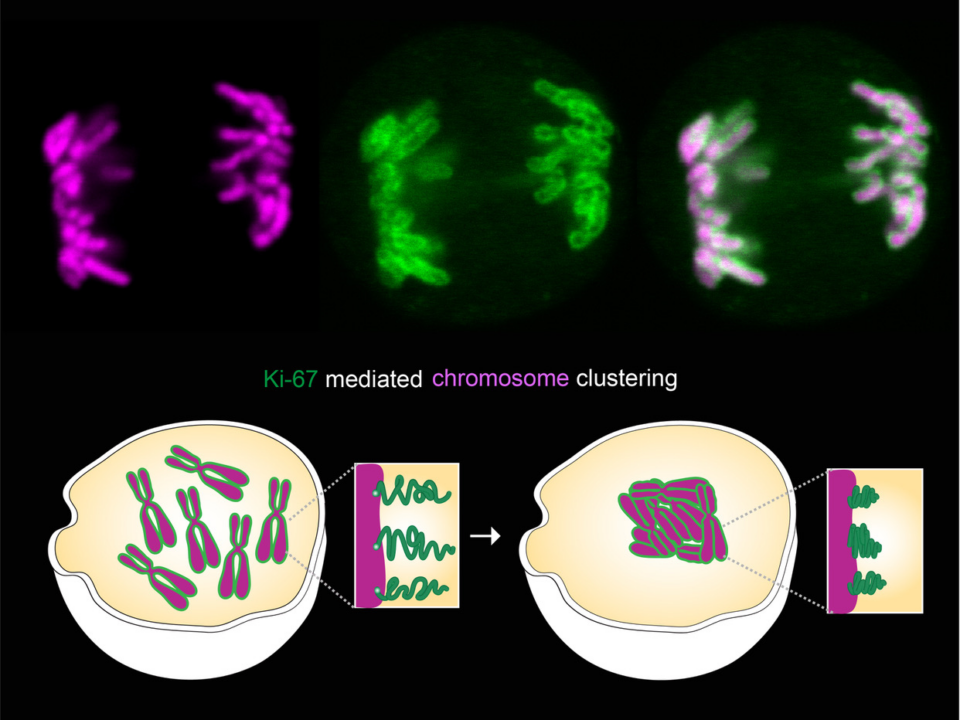
- Pericentric heterochromatin in mouse and Drosophila assembles via heterochromatin protein 1 (HP1) in a self-organizing manner into distinct nuclear subcompartments that are called chromocenters. During development chromocenters change their structure […]
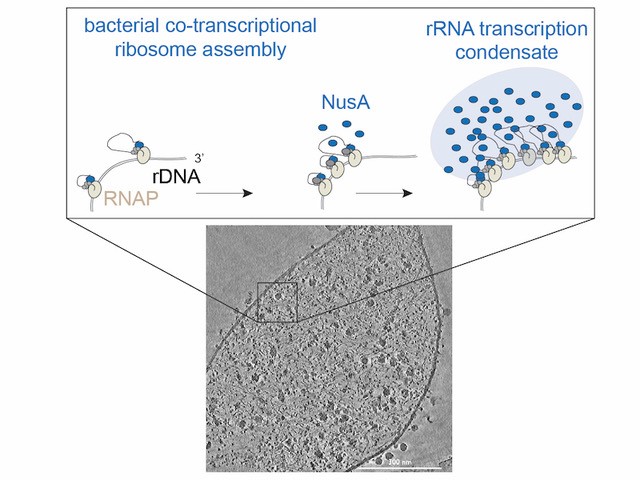
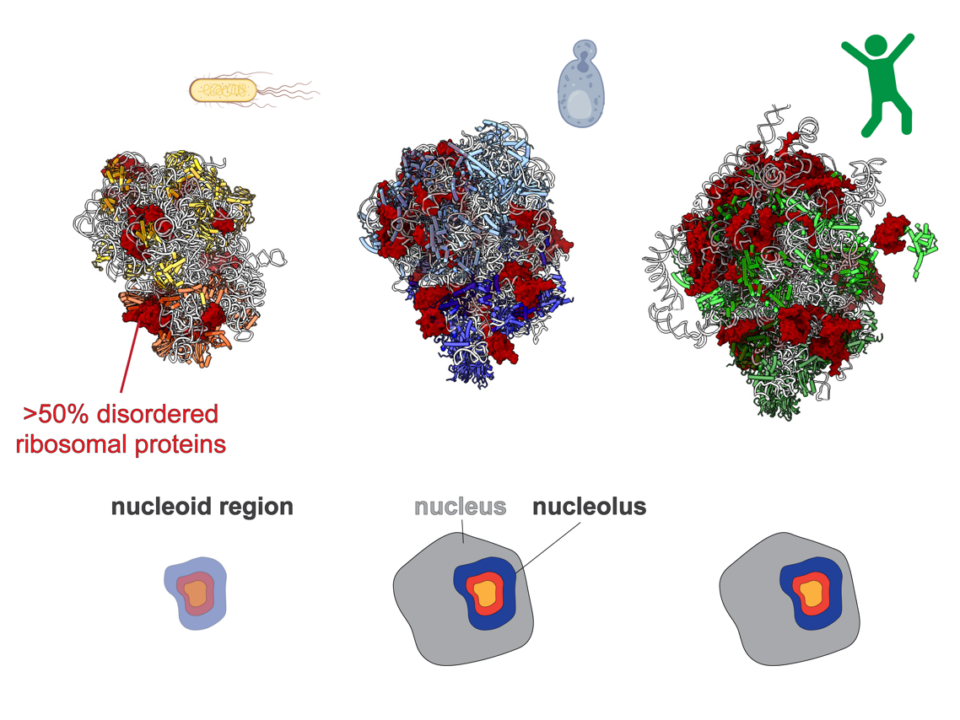
- Background: Subcellular organization via biomolecular condensation in prokaryotes has recently gained momentum with the demonstration that the fundamental process of ribosomal RNA (rRNA) transcription takes place within condensates, similar to […]
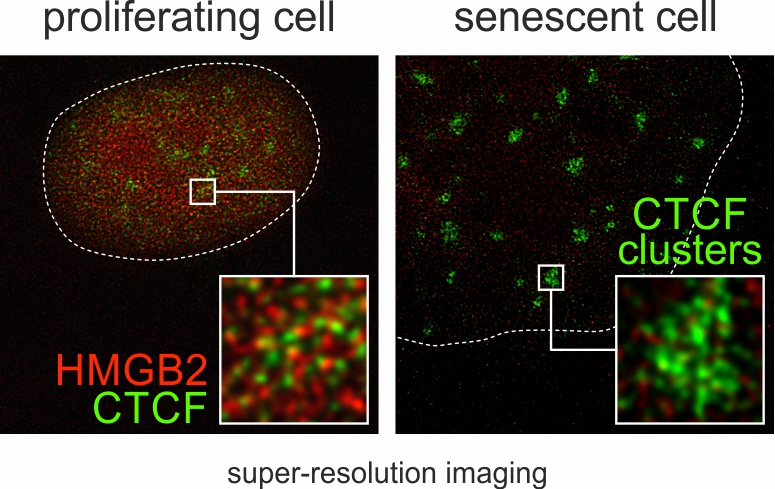
- Background: Exhaustion of the replicative potential of human cells signals their commitment to an irreversible state of cell cycle arrest known as senescence. Entry into senescence, where cells remain metabolically […]
- The formation of membrane-less cellular compartments by liquid-liquid phase separation (LLPS) relies on the unique physico-chemistry of molecules. These biomolecular condensates can separate and concentrate molecules within the cell, creating […]
- The Alberti and Seidel teams will join forces to investigate the conformational dynamics of Fused in Sarcom (FUS) in vitro and in living cells using cutting-edge single molecule technology such […]
- Recent studies have demonstrated that membrane-less condensates assemble by liquid-liquid phase separation of proteins that share multivalency, intrinsic disorder and low complexity sequences as common characteristics. The details of the […]
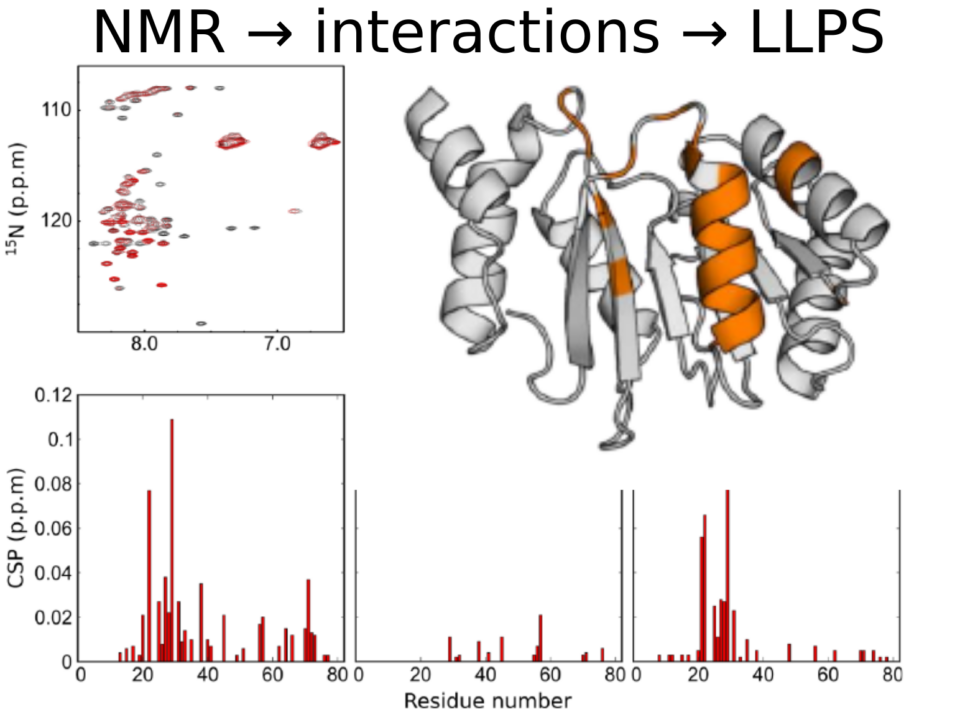
- Phase separation is considered a crucial process in disease progression, since proteins involved in amyloid-associated pathologies, like FUS, play also a crucial role in phase separation. FUS contains, like other […]
- Small RNA silencing serves to regulate genes but also as a defense against pathogens and selfish genetic elements. In insects, a specialization of small RNAs and their biogenesis factors for […]
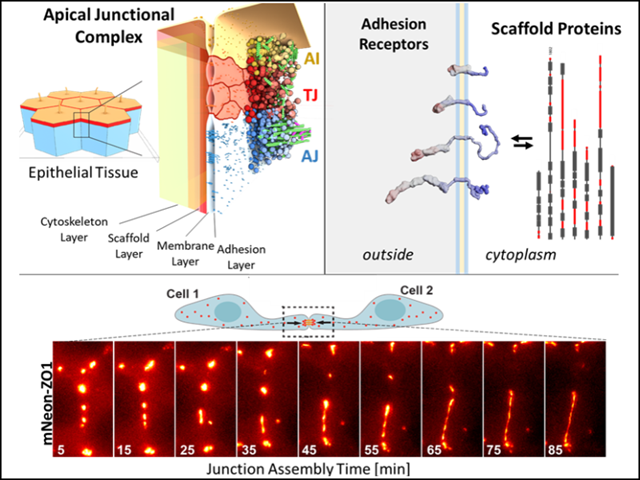
- In this project we aim to understand how tight junctions (TJ) assemble into a continuous sub-apical belt in epithelial cells. Based on cell biological and in vitro data we hypothesize […]
- Processing-bodies (P-bodies) contain the machinery that is involved in 5ʼ to 3ʼ mRNA degradation. This includes the DEAD box RNA helicase Dhh1 as well as the mRNA decapping enzyme Dcp2, […]
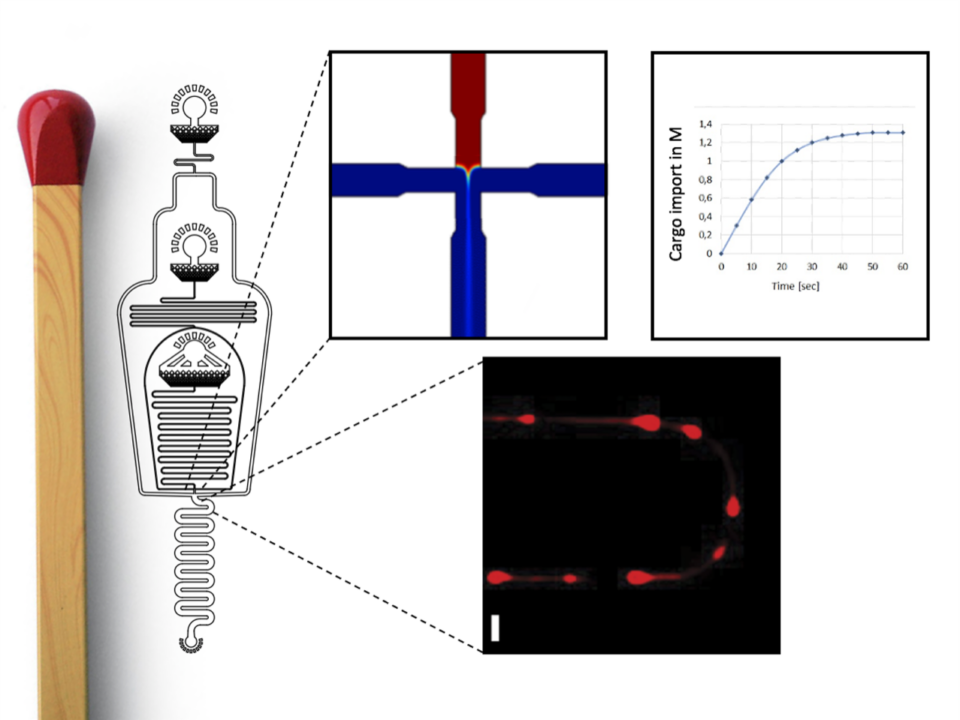
- The nuclear pore complex (NPC) is a ~120 MDa complex built from multiple copies of ~30 different nucleoporins (Nups). The NPC traverses the nuclear envelope and functions as “gatekeeper” for […]
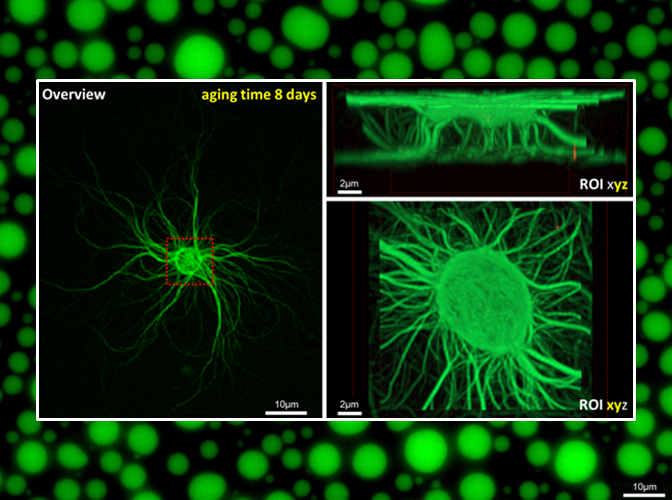
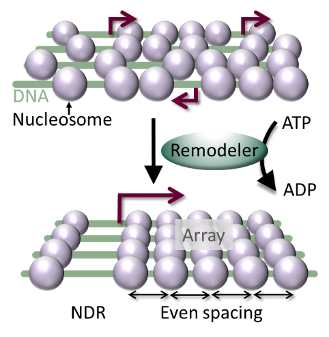
- The Mueller-Planitz lab studies the core components of chromatin – the nucleosomes and the machinery that places them in the genome. Nucleosomes are crucial to human health. Aging, for instance, […]