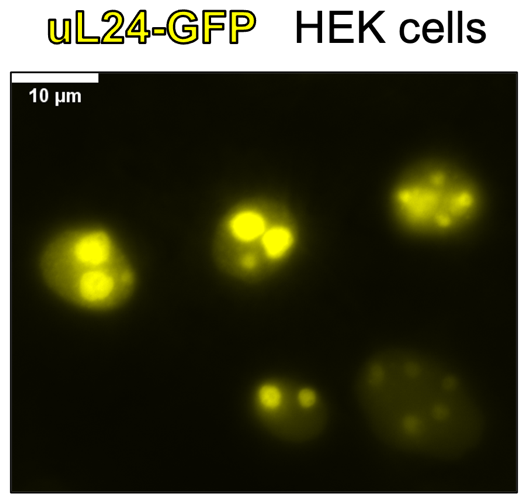
The formation of membrane-less cellular compartments by liquid-liquid phase separation (LLPS) relies on the unique physico-chemistry of molecules. These biomolecular condensates can separate and concentrate molecules within the cell, creating an optimal environment for biological reactions and assemblies. Cellular LLPS is a property of the nucleolus, a sub-compartment within the nucleus without its own membrane. The core biological function of the nucleolus is ribosome assembly, and ribosomal proteins are the most abundant proteins in the nucleolus. Strikingly, while LLPS is known to organize nucleolar proteins, whether ribosomal proteins undergo LLPS during ribosome assembly is unknown.
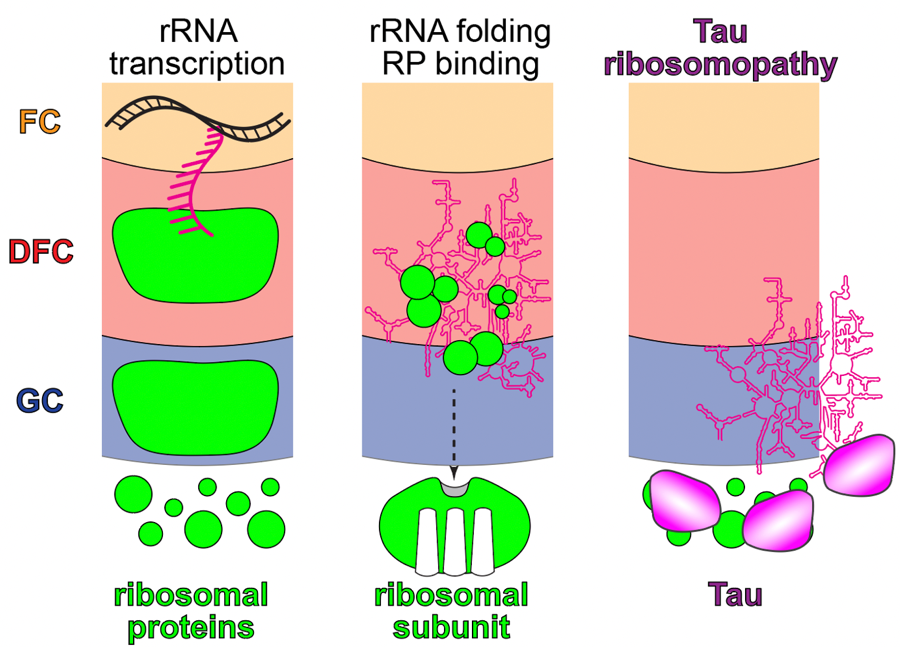
This collaborative project investigates liquid-liquid phase separation (LLPS) as a driver of ribosome assembly across evolution and ribosome regulation in neurodegenerative disease.