Background:
Plants as sessile organisms cannot run when challenged by environmental stress, including temperature stress. Instead, they have developed a plethora of sophisticated physiological and molecular reactions to maintain homeostasis. How important phase separation is for plants is just beginning to emerge. We found that the chloroplast RNA binding protein CP29A is capable of phase separation in vivo and in vitro. Phase separation is induced by low temperatures, is fully reversible after return to ambient temperature and depends on the presence of CP29A’s prion-like domain (PLD). Disruption of CP29A or deletion of the PLD leads to leaf bleaching and compromised photosynthetic activity in the cold (Fig. 1). eCLIP and RBNS experiments identified multiple chloroplast RNA targets of CP29A, including the rbcL mRNA. Mutants of CP29A show a strong reduction of rbcL mRNA in the cold. NMR analyses of recombinant CP29A during cooling unraveled a pronounced structural shift with in the PLD, indicative of a strong reduction in protein mobility resulting from molecular interactions in the condensed phase (Fig. 2). Moreover, NMR analysis of CP29As two individual RRM motifs identified surprising differences in their ability to bind RNA. Encouraged by our findings with CP29A, we asked whether other chloroplast RBPs are also capable of LLPS. Using a combination of in silico predictions, in vivo transient expression analysis and in vitro LLPS assays, we present evidence for a handful of additional chloroplast RNA binding proteins capable of LLPS.

Phenotype of cp29a null mutants after 14 days cold treatment at 8°C relative to a wild type control.
Aim:
- A detailed analysis of the biochemical and biophysical properties of LLPS shown by CP29A and (ii)
- General characterization of the function of LLPS for the additional chloroplast RNA binding proteins residing in granules discovered here.
For these goals, we bring together our complementary expertise in plant genetics and plant RNA biology (Schmitz-Linneweber lab) with expertise in the analysis of macromolecule structure, dynamics and molecular interactions (Sattler lab). We believe that a strength of our approach is that we link detailed biophysical and biochemical exploration of LLPS with molecular and in particular organismic phenotypes for plants.
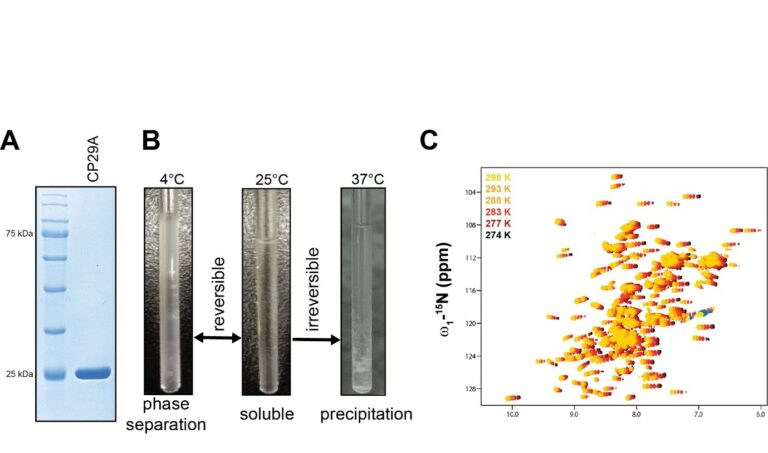
Analysis of phase separation of CP29A in vitro
A. Coomassie stain of recombinant, purified RRM1-PLD-RRM2 construct of CP29A from E. coli separated by 12% SDS-PAGE. The protein was purified via affinity tag chromatography followed by ion exchange and gel filtration chromatography.
B. NMR samples, showing the temperature dependent phase behavior of CP29A (RRMs with PLD linker) in solution. The sample is soluble at 25˚C and turns cloudy at 4˚C, while it precipitates at 37°C. Buffer composition: 20mM NaPO4 (pH 7.4), 100 mM NaCl, 1 mM DTT, 90 μM protein concentration.
C. Overlay of 1H,15N HSQC spectra of RRM1-PLD-RRM2 region of CP29A at different temperatures, colored from yellow, orange, red to black from 25˚C to 2˚C. Yellow and black spectra correspond to the soluble and dense phase, respectively.
The Team:

Michael Sattler
TUM München
Institute of Structural Biology
Helmholtz Zentrum München
Ingolstädter Landstr. 1
85764 Neuherberg, DE
Chair Biomolecular NMR-Spectroscopy
Bayerisches NMR-Zentrum, Dept Chemie, TU München
Lichtenbergstr. 4
85747 Garching, DE

Christian Schmitz-Linneweber
Humboldt-University of Berlin
Molecular Genetics Group
Institute of Biology, Faculty of Life Sciences
Philippstr. 13, Bldg. 22
Rhoda-Erdmann-Haus
10115 Berlin, DE

