Small RNA silencing serves to regulate genes but also as a defense against pathogens and selfish genetic elements. In insects, a specialization of small RNAs and their biogenesis factors for these tasks can be observed. For siRNAs, long double-stranded RNA is cleaved by Dicer-enzymes into of 21 nt long siRNAs, which are then loaded and serve as single-stranded specificity subunits in effector proteins of the Argonaute-family. In contrast, miRNAs are processed out of transcripts that fold into hairpin-structures. Since RNA interference is a major antiviral defense pathway, functional separation and “insulation” of miRNA- and siRNA-specific biogenesis factors is important to ensure a strong and rapid response to pathogens. Liquid-liquid phase separation (LLPS) appears to play an important role in this and miRNA associated factors, which regulate gene expression, associate with P-bodies (a membrane-less organelle). It is currently unclear whether a corresponding organelle exists for siRNAs.
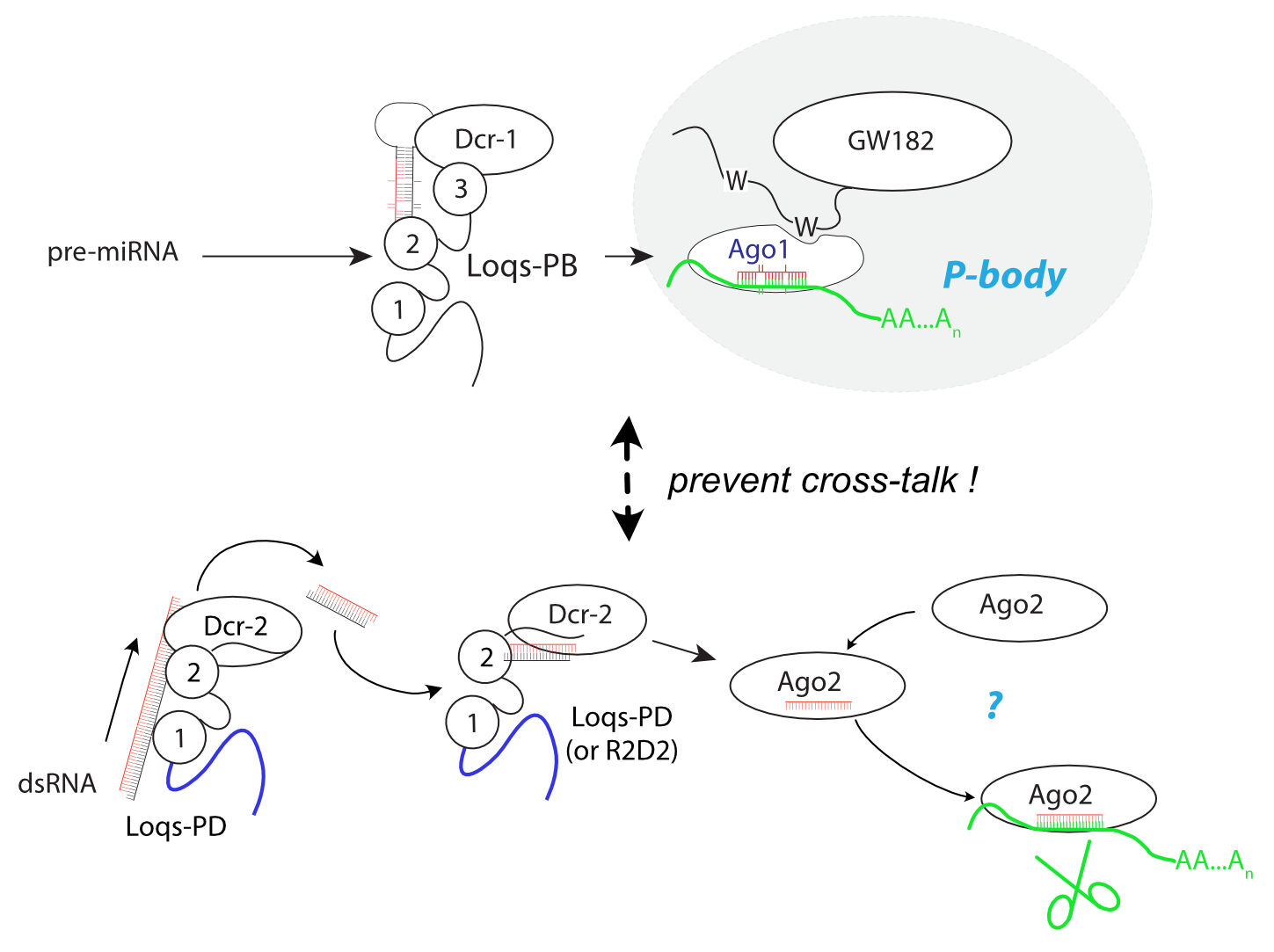
LLPS-induced membrane-less organelles provide spatially confined privileged conditions. In the condensed phase, the functionally relevant proteins/RNA/DNA are highly concentrated and can thus achieve complex reactions with high efficiency and specificity. The liquid-like nature of condensates further allows dynamic exchanges of different components in and out of the condensates.
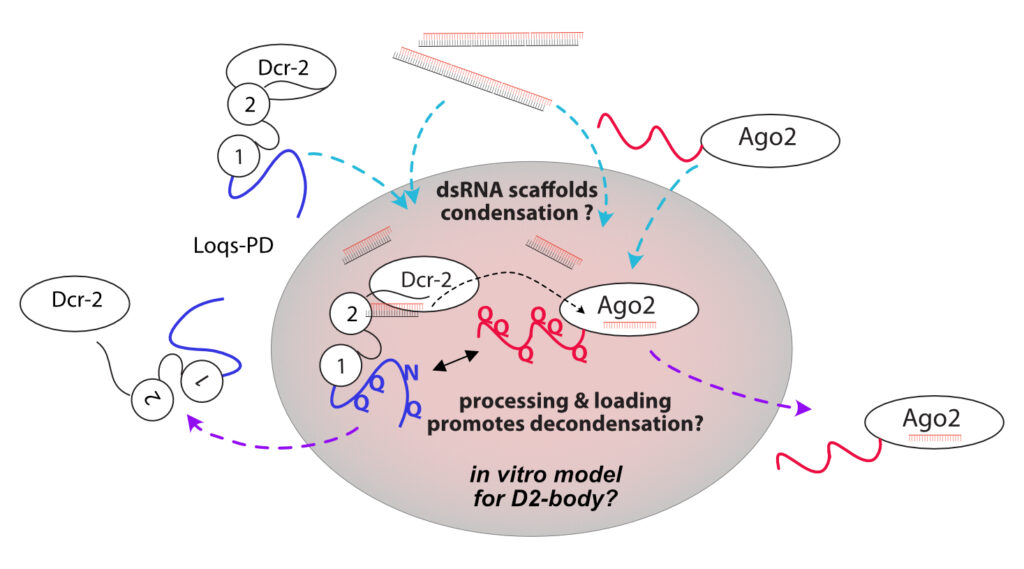
We are investigating molecular mechanisms of LLPS that foster the biogenesis of siRNAs in Drosophila melanogaster. Specifically, we want to understand the interplay between instrinsically disordered, low complexity regions in the Ago2 and Loqs proteins and their binding to double-stranded RNA. How does this lead to condensate formation, and how does it affect substrate transformation? Combining both in vitro and in vivo approaches, we will identify the key regions/residues that mediate phase separation. We complement this with experiments of proteins containing mutations at strategic sites identified by our biophysical and cell biology studies. We will survey the resulting silencing activity with reporter assays in cells and transgenic flies. In addition, small RNA profiles obtained via deep sequencing can reveal a comprehensive picture of the resulting biogenesis and loading deficiencies.
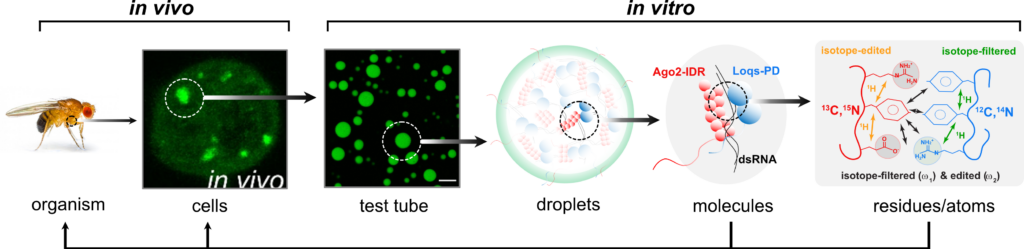
The support of SPP2191 will enable us to address questions:
• Which molecular interactions, chemical and structural features in Loqs-PD and Ago2 mediate phase separation via dsRNA and client partitioning in vitro?
• How is a specific flux of dsRNA and/or siRNAs into the condensate achieved?
• How do these molecular features relate to condensate formation in vivo?
• Are there additional factors that enter the phase separated droplets in vivo?
• How does the phase separation affect silencing efficiency and small RNA profiles?
The Team:

Clara Hipp

Selina Mußgnug

Hyun-Seo Kang

Klaus Förstemann
Klaus Förstemann has a background in the genetics and biochemistry of small RNA silencing, besides that we have a “normal fly lab”. In addition, the team works a lot with cultured fly cells. One of our strengths is the application of genome editing techniques to recapitulate genetic approaches, but also augment biochemical functionality of the proteins we study.
Ludwig-Maximilians-Universität München
Gene Center and Department of Biochemistry
Feodor-Lynen-Straße 25
81377 München

Michael Sattler
Michael Sattler and his team combing integrative structural biology, in particular solution NMR, and biophysical techniques, to study the structure, dynamics and molecular interactions of proteins and RNAs. Solution techniques provides unique information about conformational dynamics. To characterize molecular details linked to LLPS, they will combine solution and solid-state NMR with biophysical measurements to capture the conformational state and transitions involved at a molecular level.
Technische Universität München
Lehrstuhl für Biomolekulare NMR-Spektroskopie
Lichtenbergstr. 4, 85748 Garching b. München

