Aberrant liquid-to-solid phase transitions of RNA-binding proteins (RBPs) with prion-like low complexity domains (LCD) are thought to underlie the formation of pathological RBP aggregates in a number of neurodegenerative diseases, most notably amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD). In most ALS and FTD cases, the aggregating RBP is TDP-43, a ubiquitously expressed nuclear protein involved in multiple steps of RNA processing. Mutations in TDP-43 cause familial ALS and are mostly clustered in the C-terminal LCD.
Aggregated TDP-43 is abnormally post-translationally modified in ALS and FTD patients, e.g. by phosphorylation and N-terminal truncation. How disease-linked LCD mutations and abnormal post-translational modifications (PTMs) affect LLPS or aberrant liquid-to-solid transitions of TDP-43 is so far not known. Moreover, it is unclear how altered phase separation behavior of TDP-43 affects its physiological functions, its intracellular trafficking and the dynamics of TDP-43-containing membrane-less organelles, e.g. stress granules (SG). This proposal aims at (1) deciphering how disease-linked changes in the protein sequence of TDP-43 affect its phase transition behavior and (2) elucidating the cellular consequences of such altered phase transition behavior.
Current State of Research
We have gained basic insights into the molecular driving forces of TDP-43 phase separation, by introducing defined point mutations in the N- and C-terminal regions of TDP-43 that alter its phase behavior. Moreover, we found that Casein kinase 1DELTA-mediated TDP-43 hyperphosphorylation or C-terminal phosphomimetic mutations reduce TDP-43 phase separation and aggregation and render TDP-43 condensates more liquid-like and dynamic. Multi-scale molecular dynamics simulations reveal reduced homotypic interactions of TDP-43 low-complexity domains through enhanced solvation of phosphomimetic residues. Cellular experiments show that phosphomimetic substitutions do not affect nuclear import or RNA regulatory functions of TDP-43, but suppress accumulation of TDP-43 in membrane-less organelles and promote its solubility in neurons. We speculate that TDP-43 hyperphosphorylation may be a protective cellular response to counteract TDP-43 aggregation.
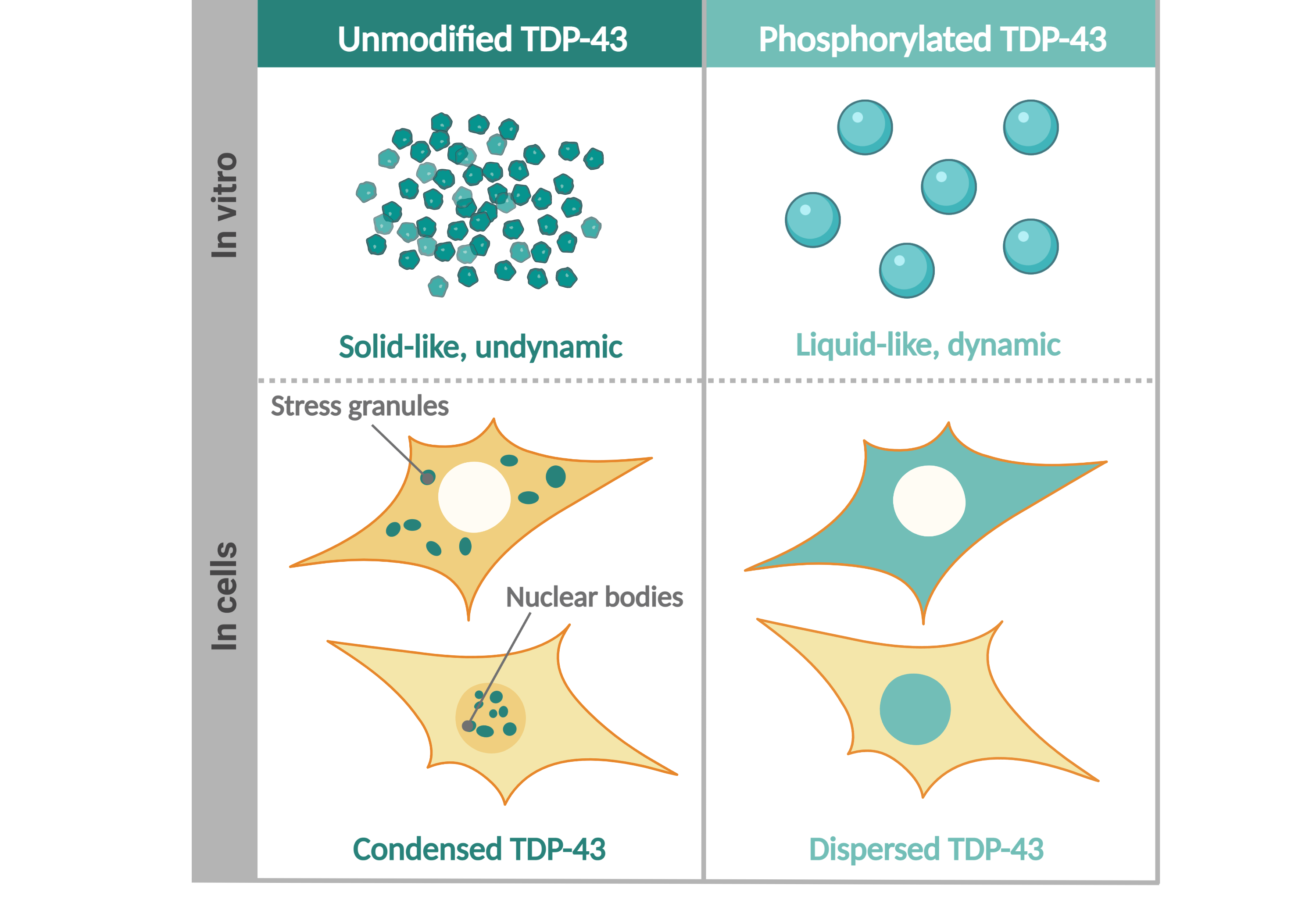
Figure: C-terminal hyperphosphorylation of TDP-42, a pathological hallmark of several neurodegenerative disorders, enhances the liquidity of TDP-43 condensates and suppresses TDP-43 condensation and aggregation, shedding a new light on this disease-linked post-translational modification.
Ref: Gruijs da Silva LA, Simonetti F, Hutten S, Riemenschneider H, Sternburg EL, Pietrek LM, Gebel J, Doetsch V, Edbauer D, Hummer G, Stelzl LS, Dormann D. Disease-linked TDP-43 hyperphosphorylation suppresses TDP-43 condensation and aggregation. EMBO J, in press
read on bioarxiv
Dorothee Dormann
The Dormann lab is focused on the role of RNA-binding proteins in neurodegenerative diseases, especially amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD). We are especially interested in two nuclear RNA-binding proteins (FUS and TDP-43), which are genetically linked to ALS and FTD and accumulate in aberrant cytoplasmic aggregates in the brains of ALS and FTD patients. Our previous work has established that neurodegeneration in ALS/FTD patients is driven by defects in 1) nucleocytoplasmic transport 2) phase separation and 3) post-translational modifications of disease-linked RNA-binding proteins. Using biochemical and cell biological approaches, we investigate how these three mechanisms are normally regulated and how they are disturbed in disease.
In particular working on the project:
Lara Silva (PhD Student)
Adjunct Director Institute of Molecular Biology
Faculty of Biology
Johannes Gutenberg University Mainz
Hanns-Dieter Hüsch-Weg 17
55128 Mainz, Germany

