Intracellular membraneless organelles (MOs) form dynamically and thermodynamically reversible via liquid-liquid phase separation (LLPS). The molecular processes that drive MO formation are largely unknown, but recent deletion experiments showed that their formation and function is often associated with intrinsically disordered protein domains. Their flexibility and ability for multiple weak protein-protein interactions (PPIs) seem to be crucial to form a protein-rich separated phase. Stress granules (SGs) are MOs that are formed in response to heat, cold, or oxidative cell stresses. They contain pathogenic forms of proteins that are associated with neurodegenerative diseases su as the enzyme superoxide dismutase (SOD1) involved in familiar amyotrophic lateral sclerosis. Pathogenic forms of SOD1 are destabilized compared to the wildtype by point mutations, show an increased propensity to aggregate, and can transform SGs into an aberrant solid-like state. In the proposed research, we want to understand how the processes of SOD1 unfolding, misfolding and aggregation are coupled to SG formation in health and disease conditions by a complementary in vitro, in vivo and in silico study. We focus on the early unfolding events of SOD1 and propose that the unfolded state strengthens the PPI network to control SG formation. The results could provide a fundamentally new understanding of the function of SGs in stress and diseas conditions associated with protein misfolding. Our research includes the development of a novel method to quantify how conformational changes of biomolecules contribute to control LLPS.
Current State of Research
Recently, we constructed a SOD1 folding reporter, including 10 disease-related mutants, and studied the respective in-cell folding stability. Based on this work, we will establish a thermodynamic and a kinetic assay to simultaneously measure SOD1 folding and SG formation in single cells. These assays will provide quantitative read-outs of how unfolded and misfolded states of SOD1 participate in PPIs controlling SG formation. To interpret the kinetic and thermodynamic data on the molecular level, we will reconstitute the system in vitro, studying varying concentrations of cosolutes. Finally, we study cellular SOD1 folding and SG regulation by biological factors as well as physicochemical factors (e.g. chaperons or osmotic pressure). We are excited to collaborate with experts in SPP 2191 to unravel the molecular details and functional aspect of LLPS. We further contribute a novel functional assay that can be used to study a broad range of molecular conformational switches that control LLPS in cells and multicellular organisms.
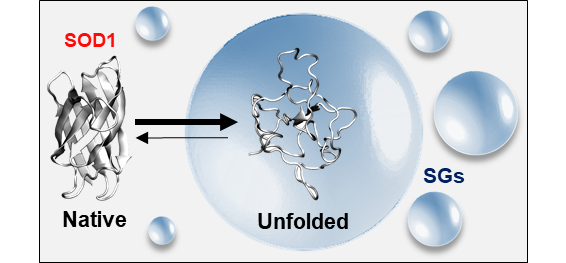
Unfolded state of destabilised ALS-related SOD1 mutant accumulates inside SGs
Simon Ebbinghaus
Simon Ebbinghaus received his doctoral degree (Dr rer. nat.) from the Ruhr-University Bochum in 2007 under the supervision of Martina Havenith and worked as a Feodor Lyne Research Fellow with Martin Gruebele at the University of Illinois (Urbana-Champaign) from 2008 to 2010. He was appointed as an Assistant Professor at the Ruhr-University Bochum in 2011 and Associate Professor in 2017. In April 2018 he moved to the Technical University Braunschweig as a Full Professor.
In particular working on this project: Sara Ribeiro and Dr. Nirnay Samanta.
Technische Universität Braunschweig
BRICS / Institut für Physikalische und Theoretische Chemie
Rebenring 56
38106 Braunschweig
Dr. Nirnay Samanta and Sara Ribeiro short CVs:
Nirnay did his doctorate in the S.N. Bose National Centre for Basic Sciences, India, receiving his doctoral degree in Chemistry in 2018, under the supervision of Rajib Kumar Mitra. During this time, he developed a strong expertise on protein folding stability and hydration dynamics of biomolecules in the test tube, using fluorescence and terahertz spectroscopy. Sara did her undergraduate and Master studies in Biochemistry at the University of Minho, Portugal. Both join in Simon´s lab in 2018 for Post-Doctorate and Doctorate, respectively, eager to investigate the connection between protein conformational changes and liquid-liquid phase separation inside living cells.
Related Publications
Samanta, N. et al. (2021) Sequestration of Proteins in Stress Granules Relies on the In-Cell but Not the In Vitro Folding Stability. J. Am. Chem. Soc. 143, 19909–19918
Hautke, A.C. and Ebbinghaus, S. (2021) Folding Stability and Self-Association of a Triplet-Repeat (CAG)20 RNA Hairpin in Cytomimetic Media. ChemSystemsChem 3, e2000052
Ribeiro, S.S. et al. (2019) The synergic effect of water and biomolecules in intracellular phase separation. Nat Rev Chem 3, 552–561
Ribeiro, S. et al. (2018) Protein folding and quinary interactions: creating cellular organisation through functional disorder. FEBS Letters 592, 3040–3053

